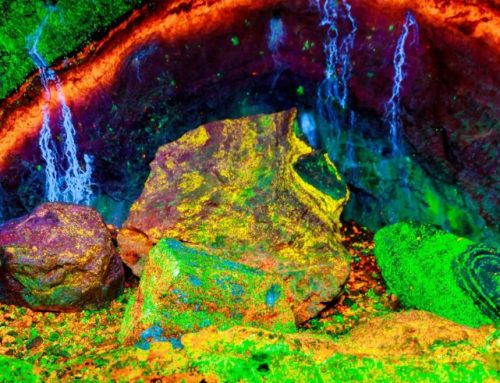
Minerals glowing under UV light is a fascinating phenomenon known as fluorescence. When exposed to ultraviolet light, certain minerals emit visible light, creating bright and vibrant colors. This article delves into why some minerals glow, the science behind fluorescence, and which types of UV light cause different effects. We’ll also explore a comparison of fluorescent and non-fluorescent minerals, and how factors like impurities and crystal structure play a role.
1. The Science Behind Fluorescence in Minerals

Fluorescence occurs when a mineral absorbs UV light and then emits visible light as electrons return to their original energy levels. The emitted light is often a different color than the absorbed UV light, producing the glow that we see.
Key Steps in Fluorescence:
- Absorption: UV light excites electrons in the mineral.
- Transition: Excited electrons move to higher energy levels.
- Emission: As electrons return to lower levels, they release energy as visible light.
Summary: The glow is a result of electrons releasing energy in the form of visible light after being excited by UV radiation.
2. Types of UV Light and Their Effects
There are three main types of UV light that influence mineral fluorescence:
| Type of UV Light | Wavelength Range | Effect on Minerals |
|---|---|---|
| UV-A (Longwave) | 320–400 nm | Common in UV flashlights, causes soft glow. |
| UV-B (Midwave) | 280–320 nm | Rarely used, can cause moderate fluorescence. |
| UV-C (Shortwave) | 100–280 nm | Most effective for bright fluorescence in minerals. |
Recommendation: For mineral enthusiasts, a UV-C (shortwave) flashlight is ideal due to its ability to reveal vibrant fluorescence.
3. Why Only Some Minerals Glow: The Role of Activators
Not all minerals fluoresce under UV light. The presence of certain activator elements is essential for fluorescence to occur.
| Activator Elements | Examples of Minerals | Fluorescent Colors |
|---|---|---|
| Manganese (Mn2?) | Calcite, Rhodonite | Red, pink, or orange |
| Uranium (UO2?) | Autunite, Uranophane | Green or yellow-green |
| Lead (Pb2?) | Willemite, Scheelite | Blue or green |
| Rare Earth Elements (REEs) | Fluorite, Apatite | Blue, green, or yellow |
Summary: The presence and type of activators significantly affect the fluorescence color and intensity.
4. The Role of Crystal Structure in Fluorescence
Crystal structure impacts how UV light is absorbed and re-emitted:
- Highly ordered crystals: More likely to fluoresce due to uniform paths for electron movement.
- Disordered or impure crystals: Less likely to fluoresce effectively.
| Crystal Type | Fluorescence Likelihood | Examples |
|---|---|---|
| Cubic (Isometric) | High | Fluorite, Halite |
| Hexagonal | Moderate | Apatite, Beryl |
| Amorphous | Low | Opal, Obsidian |
Conclusion: Minerals with more structured and uniform crystals exhibit stronger fluorescence.
5. Comparison of Fluorescent vs. Non-Fluorescent Minerals

| Aspect | Fluorescent Minerals | Non-Fluorescent Minerals |
|---|---|---|
| Activator Elements | Present (e.g., Mn, Pb, REEs) | Absent or insufficient |
| Crystal Structure | Highly ordered | Often disordered |
| UV Light Reaction | Glows under UV light | No glow or very faint |
| Examples | Calcite, Fluorite, Willemite | Quartz, Feldspar, Hematite |
Summary: The presence of activator elements and crystal structure quality distinguish fluorescent minerals from non-fluorescent ones.
6. Common Fluorescent Minerals and Their Colors
| Mineral | Common Activator | Fluorescence Color |
|---|---|---|
| Fluorite | Rare Earth Elements | Blue, green, or yellow |
| Calcite | Manganese | Red, pink, or orange |
| Willemite | Zinc or Lead | Green |
| Scheelite | Molybdenum | Blue-white |
Tip: Knowing the activator can help predict the fluorescence color of a mineral.
7. Why Some Minerals Do Not Fluoresce
Non-fluorescent minerals lack the essential activator elements or have impurities that absorb UV energy without re-emitting visible light.
Common Reasons for Lack of Fluorescence:
- Absence of activators: No elements to facilitate light emission.
- Presence of quenchers: Elements like iron (Fe) can absorb UV energy without emitting light.
- Disordered crystal structure: Prevents uniform electron transitions.
Conclusion: The lack of appropriate activators or the presence of quenchers is why many minerals do not fluoresce.
8. Practical Applications of Fluorescent Minerals
| Application | Purpose | Example Minerals |
|---|---|---|
| Mining | Identifying ore bodies | Scheelite (for tungsten), Willemite (for zinc) |
| Geology and Fieldwork | Mineral identification | Fluorite, Calcite |
| Gemstone Authentication | Detecting fakes and enhancements | Diamond, Ruby |
| Collecting and Education | Display and learning | Various fluorescent minerals |
Summary: Fluorescent minerals have practical uses in mining, geology, and gemstone authentication.
9. Tips for Viewing Mineral Fluorescence
- Choose the Right UV Light:
- Preferably UV-C (shortwave) for vibrant fluorescence.
- Dark Environment:
- Perform observations in complete darkness for best results.
- Use Protective Gear:
- Avoid direct eye exposure to UV light.
- Keep Minerals Clean:
- Dust and grime can block UV light absorption.
Conclusion: Proper tools and a controlled environment enhance the visibility of mineral fluorescence.
10. Summary: Key Factors for Fluorescence in Minerals
| Key Factor | Importance | Recommendation |
|---|---|---|
| UV Wavelength | Determines visibility of fluorescence | Use 254 nm (UV-C) for best results. |
| Activator Elements | Essential for light emission | Check for Mn, Pb, or REEs. |
| Crystal Structure | Affects light absorption and emission | Prefer highly ordered crystals. |
| Absence of Quenchers | Prevents energy loss without light | Avoid minerals with high iron content. |
Conclusion: Understanding these key factors can help enthusiasts and professionals identify and appreciate the beauty of fluorescent minerals effectively.